Covalent bonds, one of the most common classes of chemical interactions, occur when two atoms share electrons, allowing each atomic partner to have a full complement of electrons. However, in chemistry, sometimes there just aren’t enough electrons to go around.
In these kinds of chemical relationships, known as “agostic interactions” or “three-center two-electron bonds,” three separate atoms share two electrons.
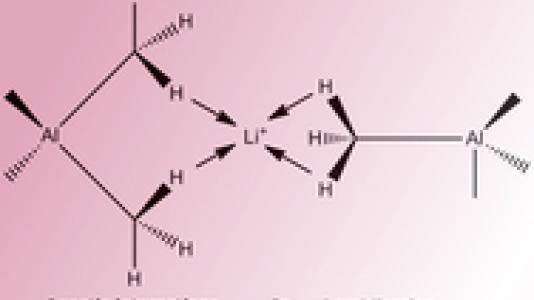
Scientists have known for some time about the presence of agostic interactions involving lithium, carbon, and hydrogen atoms, but Argonne visiting Fulbright fellow Jacqueline Cole wanted to determine what happened when an aluminum-containing fragment was appended to one of these organolithium complexes to form a lithium-aluminate compound.
“People didn’t understand how they work in terms of the reaction mechanism,” said Cole.
This lithium-aluminate molecule is one form of what chemists call “intermediates,” as they represent a middle stage between the original reactants and the reaction products. “The function of the molecule depends on what is going on in the organic part that is sandwiched between the aluminum and lithium metals. In particular, the molecular function is particularly sensitive to the behavior of the hydrogen atoms in this sandwiched organic constituent,” Cole said. “Researchers need these kinds of special intermediates to catalyze a wide range of reactions.”
Until recently, studies of the bonding in these aluminum-organolithum complexes had used X-ray diffraction, but Cole and her colleagues used neutron diffraction to characterize the bonding behavior of these lithium-aluminate complexes. “Using neutrons instead of X-rays allows you to see hydrogens much better,” she said. Neutron scattering measurements afforded researchers a three-dimensional view of the chemical bonds within the complex.
Cole determined that the lithium ion receives a negative charge from the carbon-hydrogen bond, which in turn weakens the strength of this bond. “The hydrogen ends up getting pulled towards the lithium,” Cole said.
The research appeared in the August 15 issue of Organometallics.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.