Aquatic and terrestrial environments are dynamic systems where coupled microbiological, geochemical, and hydrological processes define the complex interactions that drive the biogeochemical cycling of the major and minor elements. For example, microbial iron and sulfate reduction profoundly affect the biogeochemical cycling of carbon, iron, and sulfur in natural systems; however, the dynamics of microbial iron and sulfate reduction in the presence of both iron(III) oxides (i.e., “rust”) and sulfate (forms of iron and sulfur commonly found in nature) are not well-understood in systems with mixed microbial populations.
Researchers in the Biosciences Division and the Institute for Genomics and Systems Biology at Argonne National Laboratory used an integrated approach combining laboratory-scale model experimental systems with synchrotron-based X-ray spectroscopic techniques and high-throughput DNA sequencing to determine the response of native microbial communities in subsurface sediment from the U.S. Department of Energy’s (DOE) Integrated Field Research Challenge site in Rifle, Colo., to sulfate and specific iron(III) oxides when provided with carbon in the form of lactate, a product of microbial fermentation of biomass.
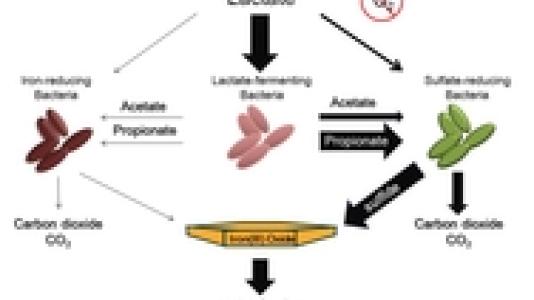
They found that instead of promoting microbial iron and sulfate reduction directly, lactate was consumed by populations of lactate-fermenting bacteria and converted to acetate and propionate. Following the fermentation of lactate, sulfate-reducing bacteria coupled the oxidation of propionate to carbon dioxide with the reduction of sulfate to sulfide. The sulfide from microbial sulfate reduction then chemically reduced the iron(III) oxides to form iron(II) sulfide, as shown in Figure 1. 16S rRNA-based microbial community analysis revealed the development of distinct communities in the presence of specific iron(III) oxides.
These results improve our understanding of the role of microbial sulfate reduction in coupling the biogeochemical cycles of carbon, iron, and sulfur while providing new insight into the effects of carbon utilization and iron(III) oxide mineralogy on microbial community development. More broadly, these results improve our understanding of complex environmental systems, which is critical for predicting the biogeochemical cycling of carbon, nutrients, heavy metals, radionuclides, and other contaminants; managing water quality; and understanding the interactions between Earth’s terrestrial and atmospheric components.
This research is part of the Subsurface Science Scientific Focus Area at Argonne National Laboratory supported by DOE’s Office of Science. Synchrotron-based X-ray analysis was performed at Argonne’s Advanced Photon Source, a national scientific user facility also supported by DOE’s Office of Science.
Kwon, M. J., M. I. Boyanov, D. A. Antonopoulos, J. M. Brulc, E. R. Johnston, K. Skinner, K. M. Kemner, and E. J. O’Loughlin. (Accepted). Effects of sulfate reduction on FeIII (hydr)oxide reduction and microbial community development. Geochimica et Cosmochimica Acta. http://www.sciencedirect.com/science/article/pii/S0016703713005462