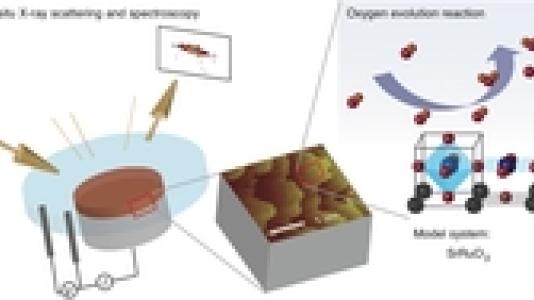
In science, like in life, finding the most effective answer to a problem often requires striking a balance.
Researchers at the U.S. Department of Energy’s Argonne National Laboratory have started to investigate the basic principles that govern the function of a group of catalysts involved in a number of different electrochemical technologies, and have found the most effective ones combine high catalytic activity with molecular stability.
“It’s clear that finding a balance between activity and stability is the key to developing materials that will be effective for a number of different applications in electrochemical systems, from batteries to fuel cells to electrolyzers,” said Nenad Markovic, Argonne Distinguished Fellow and corresponding author on the paper.
Scientists have begun to turn their focus to complex oxides as potential substitutes for traditional precious metal catalysts, like platinum, which are more reactive but simultaneously far more expensive. Identifying the optimal structure of these catalysts will have a dramatic impact on the efficiency and stability of electrochemical technologies.
“Everyone would like to replace platinum with another catalyst,” Markovic said. “But in order to do so, we have to establish some guiding principles that govern the relationship between the structure and function of these materials. This study provides the seed for determining activity-stability relationships in a wide variety of materials.”
While previous experiments tended to test the behavior of high-surface-area complex oxides, the new Argonne study on well-characterized perovskite single crystals attempted to identify the overarching fundamental mechanisms that govern the function of this class of materials in general. “In electrochemistry, nobody was looking at the structure-function relationship for complex oxides before,” Markovic said. “By characterizing these materials at atomic levels under real conditions, we can gain a better understanding of how this whole class of materials works.”
In the study, Argonne postdoctoral researcher Seo Hyong Chang saw a trade-off between a complex oxide’s stability and activity. “These oxides are kind of like tightrope walkers – the faster they ‘move,’ the less stable they are,” he said. “Our goal is to increase stability without paying the penalty in terms of activity.”
In part of the experiment, Markovic and his colleagues used Argonne’s Advanced Photon Source to get a “fingerprint” of the electronic structure of the material. They noticed that the stability of the material was closely related to the tendency of its electrons to form certain kinds of bonds. “The hope is to one day be able to do electronic manipulations to boost stability of a range of different complex oxide materials in both aqueous-based and organic-based environments,” Markovic said.
The study, “Functional links between stability and reactivity of strontium ruthenate single crystals during oxygen evolution,” appeared in the June 18 issue of Nature Communications and was funded by the U.S. Department of Energy’s Office of Science. Argonne researchers Jeff Eastman, Dillon Fong and John Freeland also contributed to the study.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The Advanced Photon Source at Argonne National Laboratory is one of five national synchrotron radiation light sources supported by the U.S. Department of Energy’s Office of Science to carry out applied and basic research to understand, predict, and ultimately control matter and energy at the electronic, atomic, and molecular levels, provide the foundations for new energy technologies, and support DOE missions in energy, environment, and national security. To learn more about the Office of Science user facilities, go to https://www.energy.gov/science/science-innovation/office-science-user-facilities.