Palladium, a precious metal closely related to platinum, is that sponge. Unlike any other element, it takes up hydrogen at room temperature and pressure. In a recent study, scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have gained new insight into how this uptake of hydrogen occurs, realized how it impacts the atomic structure of the palladium, and identified key properties of how this form of hydrogen storage could work in the future.
When hydrogen is cycled into the palladium nanoparticles, it alters and degrades the particles’ structure over time due to strain. “It’s like trying to put your foot in too small of a shoe,” said Argonne postdoctoral researcher Andrew Ulvestad, who was the study’s first author.
The ultimate goal is hydrogen storage or purification, and this research gets us closer to making that a reality.
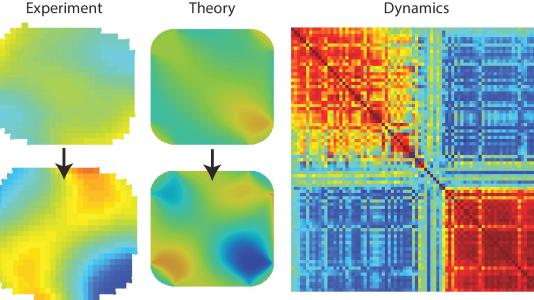
In this study, Ulvestad and his colleagues sought to map the change in the strain distribution inside the palladium nanoparticles over time. “If you can see the evolution of the strain distribution, you can tell what the hydrogen inside is doing,” Ulvestad said.
To do so, the researchers used the coherent high-intensity X-rays produced at the 34-ID-C beamline at the Advanced Photon Source, a DOE Office of Science User Facility located at Argonne.
In the nanoparticles, the researchers saw, the hydrogen atoms get incorporated into the lattice of the metal atoms that compose the nanoparticle. This, however, is not a uniform process. As the hydrogen is taken up over time, there is eventually a sudden reconfiguration of the hydrogen distribution within the lattice, a process colloquially referred to as “avalanching.”
According to Ulvestad, the “avalanches” within the nanoparticle lattice may be caused by the hydrogen atoms finding intermediate, or metastable, energy states during the reconfiguration. In large enough particles, the rearrangement of the lattice into these metastable states causes permanent defects to form in the lattice itself. As these defects form, the palladium nanoparticles become less able to take up hydrogen from the environment.
“The ultimate goal is hydrogen storage or purification, and this research gets us closer to making that a reality,” Ulvestad said. “This work may not be ready to translate to cars quite yet, but there are certain applications on the grid scale that we are beginning to look at.”
A paper based on the study, “Avalanching strain dynamics during the hydriding phase transformation in individual palladium nanoparticles,” appeared in the December 11 issue of Nature Communications.
The work was funded by DOE’s Office of Science.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.