Aquatic and terrestrial environments are dynamic systems where coupled microbiological, geochemical, and hydrological processes define the complex interactions that drive the biogeochemical cycling of water and the major and minor elements. Therefore, a thorough understanding of these complex interactions is critical for predicting the biogeochemical cycling of carbon, nutrients, heavy metals, radionuclides, and other contaminants; managing water quality; and understanding the interactions between Earth’s terrestrial and atmospheric components.
It is within this context that the Argonne Subsurface Science Focus Area (SFA), supported by the U.S. Department of Energy’s Subsurface Biogeochemistry Research Program, seeks to understand the coupled biotic-abiotic processes and the role they play in controlling the biogeochemical cycling and molecular scale transformations of iron and minor elements, as well as the subsequent role of these coupled biotic-abiotic interactions in the molecular-scale transformations of contaminants such as uranium and mercury and the biogeochemical cycling of carbon.
Iron (Fe) is the fourth most abundant element in Earth’s crust, where it is often present in the form of Fe(III) oxides, more commonly known as rust. This iron is easily converted among several chemical forms by microbes living in subsurface environments. These forms can in turn influence the immobilization and release of contaminant metals such as uranium as well as sequestration of carbon. Predictive understanding of the processes involved in these transformations is limited by lack of knowledge of the impact of many other chemical species commonly found with iron in the environment.
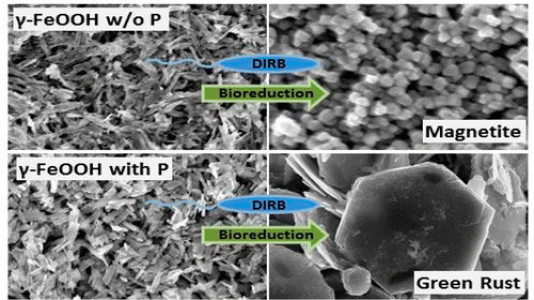
New research has provided knowledge of how phosphate ion incorporated in iron-containing minerals impacts the speciation of iron and cycling of carbonate ion (a common form of carbon in the subsurface). Scientists at Argonne National Laboratory, collaborating with the University of Iowa, Pennsylvania State University and Hamilton College, determined that phosphate bound or occluded within the Fe(III) oxides has a significant impact on minerals produced by the iron-reducing bacterium Shewanella putrefaciens CN32 (a microbe commonly found in aquatic and terrestrial environments).
In the absence of phosphate the Fe(III) is largely converted to magnetite, but when phosphate is present within the Fe(III) particles, a significant amount of a reactive iron-containing species known as carbonate green rust is produced. Green rust is highly effective in reducing and immobilizing contaminants such as radionuclides and toxic metals. This study therefore provides key information for understanding how to use the bacteria to treat contaminated environments efficiently, as well as highlighting the interplay between the biogeochemical cycles of iron, phosphorous, and carbon.
Reference: O’Loughlin, E. J.; Boyanov, M. I.; Flynn, T. M.; Gorski, C.; Hofmann, S. M.; McCormick, M. L.; Scherer, M. M.; Kemner, K. M., “Effects of bound phosphate on the bioreduction of lepidocrocite (γ-FeOOH) and maghemite (γ-Fe2O3) and formation of secondary minerals”. Environmental Science and Technology 47(16):9157-9166, 2013, DOI 10.1021/es400627j. http://pubs.acs.org/doi/abs/10.1021/es400627j