A new method that could greatly simplify the reprocessing of used nuclear fuel and make its disposal safer has been developed by researchers from the U.S. Department of Energy’s Argonne National Laboratory and Pacific Northwest National Laboratory.
A significant technical challenge to closing the nuclear fuel cycle and increasing the safety of used nuclear fuel reprocessing involves separating the most radioactive elements (minor actinides) from the remainder of the waste products (lanthanides).
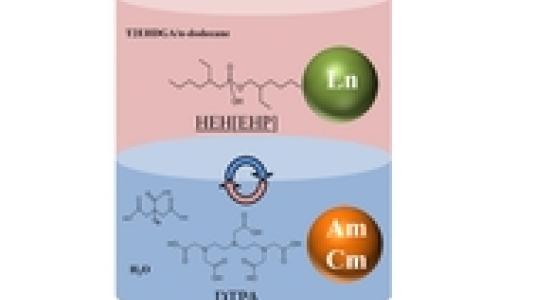
Called Actinide Lanthanide Separation Process (ALSEP), the new process separates the two materials in a single step.
ALSEP can be used in a closed nuclear fuel cycle, meaning radioactive materials are recycled and used to make new reactor fuel. Separating actinides from lanthanides is the last separation step in a series for a closed nuclear fuel cycle.
The nuclear cycle begins with the uranium fuel fabrication from the uranium ore that, once ready, is used to produce energy in the nuclear reactor. When the uranium is spent, or fully used, it goes through chemical re-processing where uranium and plutonium are separated out and can be recycled. The remaining substance is a mixture of americium, curium, lanthanides and other isotopes.
Americium and curium are minor actinides that contribute the most heat to nuclear fuel repositories after they are separated from elements like uranium and plutonium. The actinides are also extremely radiotoxic, making it desirable to remove them and handle them separately.
Current methods of separation can be too expensive, involve multiple chemical stages, and are unsafe due to the complexity of the processes. ALSEP significantly decreases the number of steps needed to perform the separation, making it more cost-effective.
“Our approach is based on simplicity,” says Artem Gelis, nuclear chemist and principal author of the study. “To get efficient separation, we use a water-soluble agent called DTPA, which takes out the americium and curium.”
Separation can happen in one of two ways, both requiring that the organic extractants involved be soluble in industrial thinning agents like kerosene and withstand hydrolysis and radiolysis.
At the end of the process, the recovered minor actinides can be fissioned in advanced nuclear reactors and the lanthanides disposed of in a safe waste repository.
The work is reported in “Actinide Lanthanide Separation Process - ALSEP,” published January 4, 2014 in the journal Industrial and Engineering Chemistry Research.
This work was supported with funding by the U.S. Department of Energy, Office of Nuclear Energy, Fuel Cycle Research and Development Project.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.