Mercury (Hg), a common pollutant released from volcanoes, mining activity, burning of fossil fuels, and other industrial and consumer applications, is a major environmental concern because of its toxicity to humans and wildlife. Mercury bioaccumulates in organisms — including humans — damaging the nervous, digestive, and immune systems, the lungs and kidneys, and potentially causing death.
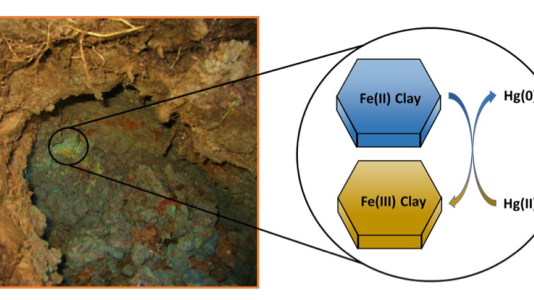
A thorough understanding of the release of volatile mercury from soils and sediments is critical; mercury can escape as a gas into the atmosphere and mobilize on a global scale. Its transformation from a form that tends to remain in the soil/sediment — mercury(II) — to a form that can escape as a gas — mercury(0) — was poorly understood until now. Scientists knew that bacteria and other microorganisms could transform mercury(II) to mercury(0), but our study revealed that the clay minerals commonly found in soils can also cause this transformation.
The reduction of mercury(II) to mercury(0) in soils and sediments is key to its distribution between the atmospheric and aquatic/terrestrial reservoirs and to the overall biogeochemical cycling of mercury. The transformations between the two forms of mercury can be caused by microorganisms or by chemical reactions.
The Argonne team used x-ray spectroscopy at the laboratory’s Advanced Photon Source to show that iron(II) in clay minerals commonly found in soils/sediments can reduce mercury(II) to mercury(0), a previously unknown process in the biogeochemistry of mercury. This finding — that clay minerals may play a role in the emission of mercury(0) from soils and sediments — can lead to improved models of global mercury cycling and better protection of human health and the environment.