Protein reagents have wide ranging applications from field-deployable biosensors and industrial processes to human therapeutics. However, the relative fragility of antibodies and enzymes severely complicates their use outside of controlled laboratory environments. The challenge is to develop protein reagents with long shelf-life and improved thermostability.
The Antibody Stabilization Pipeline
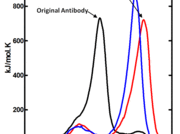
We have developed a semi-rational approach to protein stability engineering. The technique has been successfully applied to antibodies and in principle is applicable to any protein family. The foundation of the stabilization technique is the analysis of sequence variations observed in homologous protein families. Our antibody stabilization method also relies on an extensive database of immunoglobulin domain mutations that we have engineered and characterized in our laboratory. Researchers in the Biosciences have used the method to develop robust antibodies for biosensors, stabilizing antibody fragments (scFvs) to unprecedented levels (Figures 1 and 2). The method has also been applied with great success to the improvement of full-length therapeutic antibodies, generating a panel of variants of an anti-cancer antibody with a 10-11 °C higher melting temperature (Tm) compared to the wild type antibody (Figure 3).
Improving the Stability of Rubisco Activase, the Weak Link in the Biological CO2 Fixation Machinery
Rubisco, thought to be the most abundant enzyme on earth, is a plant enzyme that provides the first step in conversion of atmospheric CO2 into energy-rich sugars. Despite its critical role in carbon cycling, Rubisco is a rather slow and inefficient enzyme. Moreover, during a certain step of its catalytic cycle, Rubisco can get “stuck” in an inactive form. A chaperone-like enzyme, Rubisco activase uses an ATP-dependent reaction to convert back to its active form. The Rubisco activase of many plant species may have low thermal stability. For these plants, an increase in temperature of only a few degrees destroys the Activase, resulting in decreased Rubisco output and reduced plant growth. It has been hypothesized that as the Earth’s climate becomes warmer, labiality of Rubisco Activase could result in widespread crop losses. Research in Biosciences is producing stable variants of Rubisco Activase that can be transferred into food and biofuel crops.
Stabilized Antibody Fragments
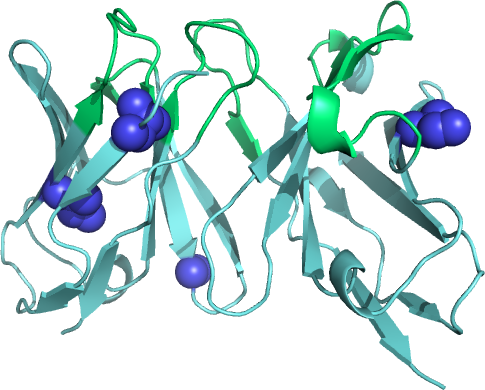
Figure 1.
Ribbon diagram of a stabilized antibody fragment (scFv) that binds the B. anthracis protein BclA. The stabilizing amino acid modifications are depicted as blue spheres.
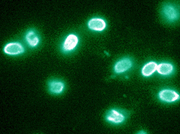
Figure 2.
Binding of stabilized scFv to B. anthracis spores. Following incubation with spores, the scFv was detected with a fluorescent reagent, Alexa Fluor 488-Protein L. (Spore binding assay was performed by Dr. Adam Driks, Loyola University Medical Center, Maywood, IL.)
Figure 3.
Thermal stability of full-length antibodies was measured by differential scanning calorimetry.