Scientific Achievement
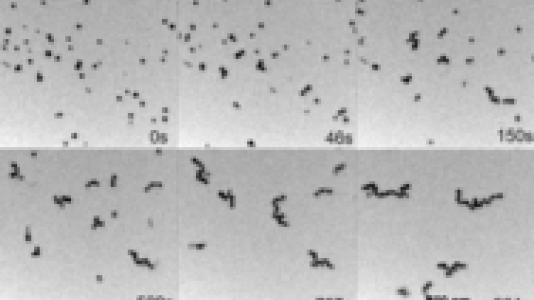
The self-assembly of gold nanoparticles coated with specific organic ions in water was observed in situ for the first time by transmission electron microscopy (TEM) using a liquid cell, as it occurs in real time. The gold nanoparticles formed one-dimensional chains within a few minutes.
Significance
The self-assembly of nanoparticles attracts intense attention for its potential application in the fabrication of hybrid systems with collective properties from different types of materials. The observations clearly elucidate the complex mechanism of charged nanoparticle self-assembly processes. They also paint a cautionary tale on using TEM in situ cells to imitate self-assembly processes in actual solution environments.
Research Details
The expertise of Argonne’s Center for Nanoscale Materials, involving in situTEM techniques, colloidal nanoparticle synthesis, zeta-potential measurements, and absorption spectra were exploited in this study.
The hydrated electrons formed in radiolysis of water decrease the overall positive charge of cetyltrimethylammonium-coated (CTA)-gold nanoparticles. The nanoparticles also were coated with negative citrate ions. The anisotropic attractive interactions, including dipolar and Van der Waals interactions, overcome the repulsion among the nanoparticles and induce nanoparticle assembly. The spatial segregation of different sizes of nanoparticles as a result of electric field gradients within the cell also was observed.
Reference
Y. Liu, X-M Lin, Y. Sun, and T. Rajh, “In Situ Visualization of Self-Assembly of Charged Gold Nanoparticles,” J. Am. Chem. Soc., 135, 3764 (2013)