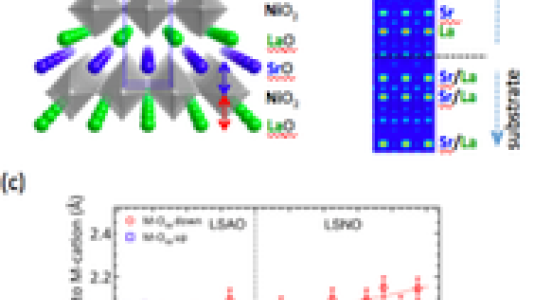
Strain is known to have profound influence on electronic and magnetic properties of complex oxides. However, epitaxial strain is limited in range, being able to create distortions in bond lengths and bond angles that are typically 10% of the equilibrium value via electrostatics. In bulk LaSrNiO4, the La and Sr cations occupy the A-site randomly, and the crystal possesses a center of inversion symmetry. We use oxide molecular beam epitaxy techniques to create an artificial polar analog of this material depositing monolayers of LaO, NiO2 and SrO in sequence, as shown in (a). An internal dipolar field is set up since the LaO carries a net (+1) charge and the NiO2 is nominally (-1). This artificial crystal is non-centrosymmetric. The cation layering can be determined by measuring crystal truncation rods and using an x-ray phase retrieval technique called ‘COBRA’, as shown in (b). LaSrNiO4 is known to be metallic. The internal dipolar electric fields in the polar analog are not entirely screened by the electrons at the Fermi level. In fact, the Ni-O bonds distort to screen these internal dipolar fields. These distortions can be larger than 10%. This in turn changes the Ni-O bond hybridization, which we have modeled using density functional theory, and measured with resonant x-ray spectroscopy.
Polar Cation Ordering: A Rout to Introducing >10% Bond Strain into Layered Oxide Films,
Brittany B. Nelson-Cheeseman, Hua Zhou, Prasanna V. Balachandran, Gilberto Fabbris, Jason Hoffman, Daniel Haskel, James M. Rondinelli, and Anand Bhattacharya,
Adv. Funct. Mater. 24, 6884 (2014)