- Model for the Fabrication of Tailored Materials for Lithium-ion Batteries (ANL-IN-10-036)
2012 R&D 100 Award Winner
The Invention
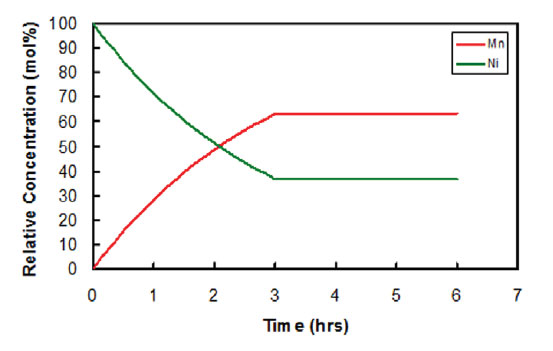
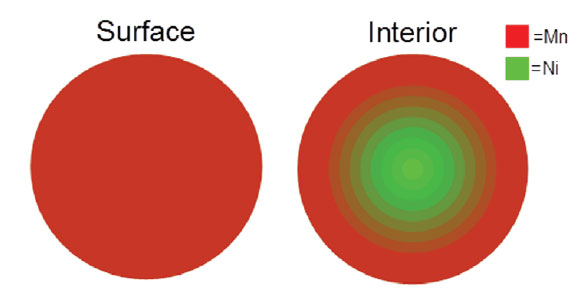
A unique method to control the composition gradient of materials in lithium-ion cathodes. The material particles created using this method are nickel-rich on the inside for a high capacity battery, and manganese-rich on the exterior surface for increased safety and stability.
The process includes combining a first transition metal compound with a second transition metal compound to form a transition metal source solution, and combining that solution with a precipitating agent to form a precursor solution. The radius of precipitating particles consists of a transition metal oxide core and at least two layers of transition metal oxide. The particles have a transition metal concentration gradient in which the ratio of the first transition metal to the second transition metal is inversely proportional to the radius of the particle over at least a portion of the radius. The transition metal used in the first and second transition metal compounds include manganese, cobalt, nickel, chromium, vanadium, aluminum, zinc, sodium, titanium or iron. The first and second transition metal compounds can also include, but are not limited to, metal sulfates, nitrates, halides, acetates or citrates.
Benefits
- Creates a gradient of different materials for increased safety and stability;
- Gradient runs throughout the entire radius of the particle;
- Particles are ideally small, 10-20 microns in size; and
- Leads to high-capacity batteries.
Applications and Industries
The particles can be used to create composite cathodes in batteries for
- Electric and plug-in hybrid electric vehicles;
- Portable electronic devices;
- Medical devices; and
- Space, aeronautical, and defense-related devices.
Developmental Stage
Reduced to practice

![Argonne’s Electrochemical Analysis and Diagnostics Laboratory (EADL) provides reliable, independent, and unbiased evaluations of battery performance and life, which serve as progress measures for DOE and U.S. Advanced Battery Consortium projects. [Image courtesy of Argonne National Laboratory.]](https://sandbox4-www.anl.gov/sites/www/files/styles/article_teaser_16x9/public/2019-12/EADL_32751D099_1920x1080.jpg?h=d1cb525d&itok=LdSfWaCk)

