Lithium-ion batteries that power today’s electric cars depend on cobalt-containing cathode materials. In turn, these materials are becoming increasingly dependent on nickel. However, due to growing concerns around the associated safety, cost, and material supply, the industry continues to search for battery chemistries that rely far more on sustainable elements such as manganese (Mn).
Although Mn has a long and storied history in energy storage, low energy densities as well as bulk and surface instabilities of Mn-rich oxides have hindered their widespread use in EV applications. Researchers at the U.S. Department of Energy (DOE)‘s Argonne National Laboratory are developing a suite of lithium-ion cathode technologies to overcome these challenges and enable substantially increased use of Mn. These technologies, which were developed through funding from DOE’s Office of Energy Efficiency and Renewable Energy Vehicle Technologies Office, could apply not only to electric vehicles but the electric grid, where battery storage is needed for variable energy resources like wind and solar, and other industries.
Building on Argonne’s pioneering efforts in the development of nickel-manganese-cobalt (NMC) cathode materials, now licensed to manufacturers worldwide, the team at Argonne is focused on higher capacity versions, stabilized through novel, bulk and surface designs.
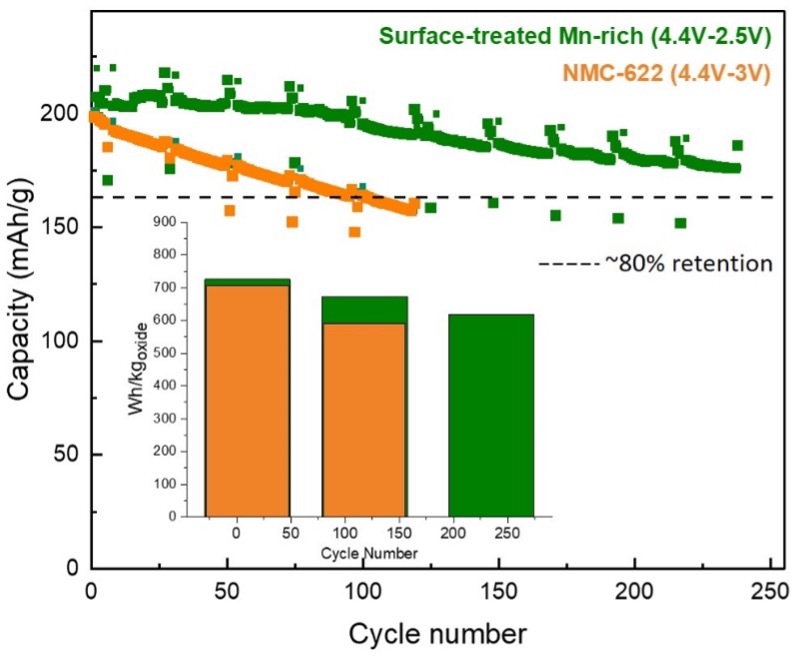
The figure in this article shows cycling data of a commercially available NMC-622 cathode compared to an Argonne cathode containing ~60% Mn and 0% cobalt. These cathode-electrodes were tested using graphite anodes under VTO’s standardized, high-voltage protocols1. These protocols are designed to stress materials and exacerbate high state-of-charge damage, particularly surface damage from electrolyte interactions. Argonne’s unique surface treatments specifically designed for Li- and Mn-rich cathodes enable ~250 high-voltage cycles with >80% retention. For comparison, the NMC-622 cycled under the same protocol reaches 80% after ~100 cycles. The inset shows the specific energy of the cathode-electrodes (Wh/kgoxide) as a function of cycling. The surface stabilized, Mn-rich electrode delivers more energy for more cycles than the NMC-622.
Argonne’s intellectual property portfolio of manganese and lithium rich materials includes a range of surface options for various NMCs as well as complex, Mn-rich cathode structures, including layered-type structures, spinel-type structures, rock salt-type structures, and combinations thereof.
1. B. R. Long et al. J. Electrochem. Soc., 163, A2999 (2016).
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.