M.M. Thackeray, M.K.Y. Chan, L. Trahey, S. Kirklin, and C. Wolverton
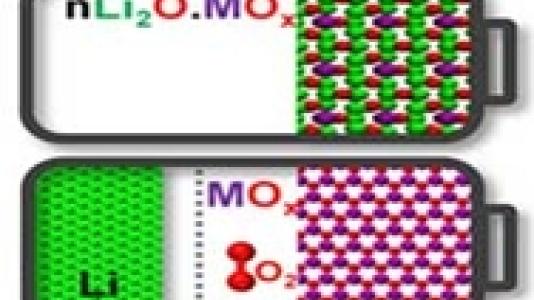
Researchers at the Center for Electrochemical Energy Science (CEES) at Argonne National Laboratory have presented a vision for designing hybrid Li-ion/Li-O2 cells that would provide an energy density significantly higher than that of conventional Li-ion cells, without the need for external oxygen.
In conventional Li-ion batteries, lithium ions shuttle between a carbon anode and a metal oxide cathode during charge and discharge, whereas Li-O2 batteries rely on electrochemical reactions involving lithium metal and oxygen gas. CEES has proposed a vision to use lithium-metal-oxide materials from which lithium and oxygen can be removed during charge, which can then recombine to form a Li-metal-oxide product during discharge, at a voltage at, or above, Li2O2 and Li2O formation. Because all the lithium, metal, and oxygen required for the reaction are all contained in a parent crystalline solid, they are called “all-in-one” electrode materials.
First-principles density functional theory calculations have confirmed experimental results and the concept that a charged product, derived electrochemically by lithium and oxygen extraction from a parent Li5FeO4 crystalline structure, can recombine (at thermodynamic equilibrium) with lithium and oxygen to regenerate the Li5FeO4 composition above 3 V during discharge, seemingly with iron ions acting to catalyze the reaction.
The work described was performed at Argonne National Laboratory and Northwestern University.
Reference
Michael M. Thackeray, Maria K. Y. Chan, Lynn Trahey, Scott Kirklin, and Christopher Wolverton, “A Vision for Designing High Energy, Hybrid Li-Ion/Li-O2 Cells,” Journal of Physical Chemistry Letters, 4, 3607 (2013). Available at http://dx.doi.org/10.1021/jz4018464.