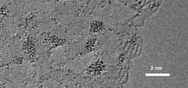
Batteries based on sodium superoxide and on potassium superoxide have recently been reported. However there have been no reports of a battery based on lithium superoxide (LiO2), despite much research into the lithium–oxygen (Li–O2) battery because of its potential high energy density. Several studies of Li–O2 batteries have found evidence of LiO2 being formed as one component of the discharge product along with lithium peroxide (Li2O2). Theoretical calculations have indicated some forms of LiO2 may have a long lifetime. These studies also suggest that it might be possible to form LiO2 alone for use in a battery. However, solid LiO2 has been difficult to synthesize in pure form because it is thermodynamically unstable with respect to disproportionation, giving Li2O2.
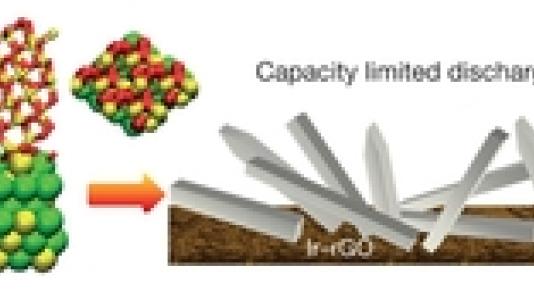
In this study users of the Center for Nanoscale Materials from Argonne’s Materials Science and Chemical Sciences & Engineering Divisions, working collaboratively with CNM staff, show that crystalline LiO2 can be stabilized in a Li–O2 battery by using a suitable graphene-based cathode. Various characterization techniques reveal no evidence for the presence of Li2O2. A novel templating growth mechanism involving the use of iridium nanoparticles on the cathode surface may be responsible for the growth of crystalline LiO2. The results demonstrate that the LiO2 formed in the Li–O2 battery is stable enough for the battery to be repeatedly charged and discharged with a very low charge potential (about 3.2 volts). This discovery may lead to methods of synthesizing and stabilizing LiO2, which could open the way to high-energy-density batteries based on LiO2 as well as to other possible uses of this compound, such as oxygen storage.
Electron microscopy was performed at the Center for Nanoscale Materials. X-ray measurements were performed at the Advanced Photon Source. Density functional calculations were performed using CNM’s Carbon cluster, ALCF’s Mira, and the LCRC Fusion cluster at Argonne.
J. Lu et al., Nature, 529, 377 (2016); doi:10.1038/nature16484
In the News