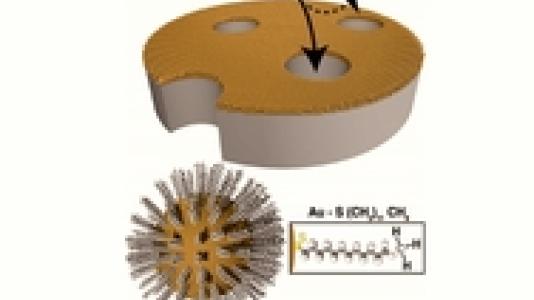
From proton exchange membranes in fuel cells to ion channels in biological membranes, the well-specified control of ionic interactions in confined geometries profoundly influences the transport and selectivity of porous materials. A new study by Center of Nanoscale Materials’ users from the University of Chicago, working with the CNM EMMD Group, describes a versatile new approach to control a membrane’s electrostatic interactions with ions by depositing ligand-coated nanoparticles around the pore entrances. Leveraging the flexibility and control by which ligated nanoparticles can be synthesized, ligand terminal groups such as methyl, carboxyl and amine can be used to tune the membrane charge density and control ion transport. Further functionality, exploiting the ligands as binding sites, is demonstrated for sulfonate groups resulting in an enhancement of the membrane charge density. The results are extended to smaller dimensions by systematically varying the underlying pore diameter.
As a whole, these results outline a previously unexplored method for the nanoparticle functionalization of membranes using ligated nanoparticles to control ion transport. While this study focuses on the introduction of charge-based interactions, ultimately the results highlight a general pathway towards membrane functionalization that utilizes ligated nanoparticles as building blocks, functionalized a-priori by a suitable choice of encapsulating ligand. This work opens up exciting possibilities for a number of functionalized components that have been chemically adsorbed onto the surfaces of nanoparticles, and for the first time, describes a means by which to deliver this functionality to porous substrates. Such an approach could have an immediate impact for wide range of membrane-based systems, including those of biological and biomimetic origin, in both fundamental scientific studies as well as applied technologies.
E. Barry et al., Nature Communications, 5, 5847 (2014).