- Containerless synthesis of amorphous and nanophase organic materials
The Invention
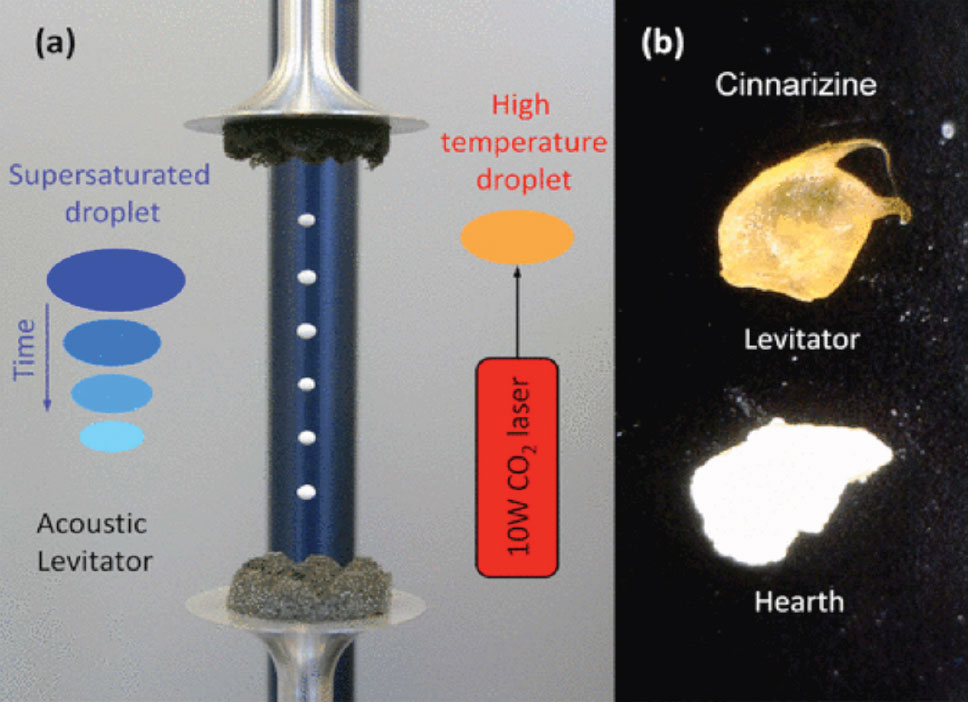
Making fast-acting pharmaceuticals is a goal of almost every drug company. The route of delivering pharmaceuticals in the form of amorphous solids has long been recognized as a possible way to improve dissolution rates and to increase both solubility and bioavailability. Development in this direction is becoming increasingly important due to the emergence of many new drugs that are virtually insoluble in their crystalline form. Researchers at Argonne National Laboratory have developed the technique of acoustic levitation to prepare amorphous solids and molecular gels that can be easily applied to the pharmaceutical manufacturing process. This technique would improve the solubility and bioavailability of several drugs.
The acoustic levitation techniques developed at Argonne keeps the drug solution from making contact with any surface whatsoever during the solvent evaporation process. This containerless process was developed and tested on several over-the-counter and prescription pharmaceuticals. Several of the pharmaceuticals amorphized using the Argonne process remained completely amorphous for four months or longer. Please review the publication for additional details.
Benefits
A containerless process:
- Precludes the possibility of a drug’s interaction with its container;
- Provides a more effective means of synthesizing amorphous pharmaceutical compounds;
- Offers higher yields than current state-of-the-art methods;
- Reduces the potential for contamination during the manufacturing process; and
- Is expected to advance the development of amorphous drug forms, increasingly important due to new drugs that are virtually insoluble in crystalline form.
Applications and Industries
Pharmaceutical industry
Developmental Stage
Proof of principle