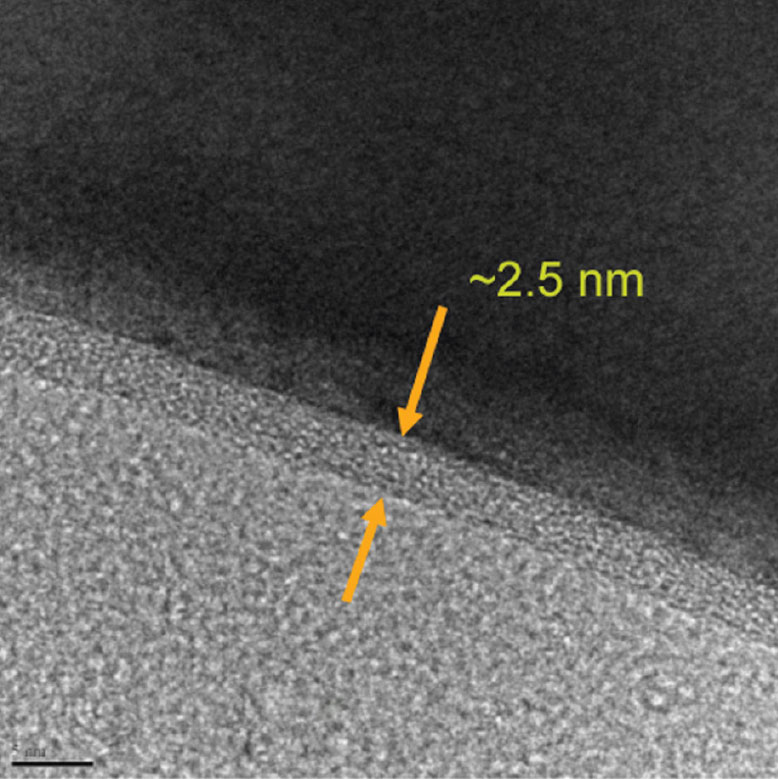
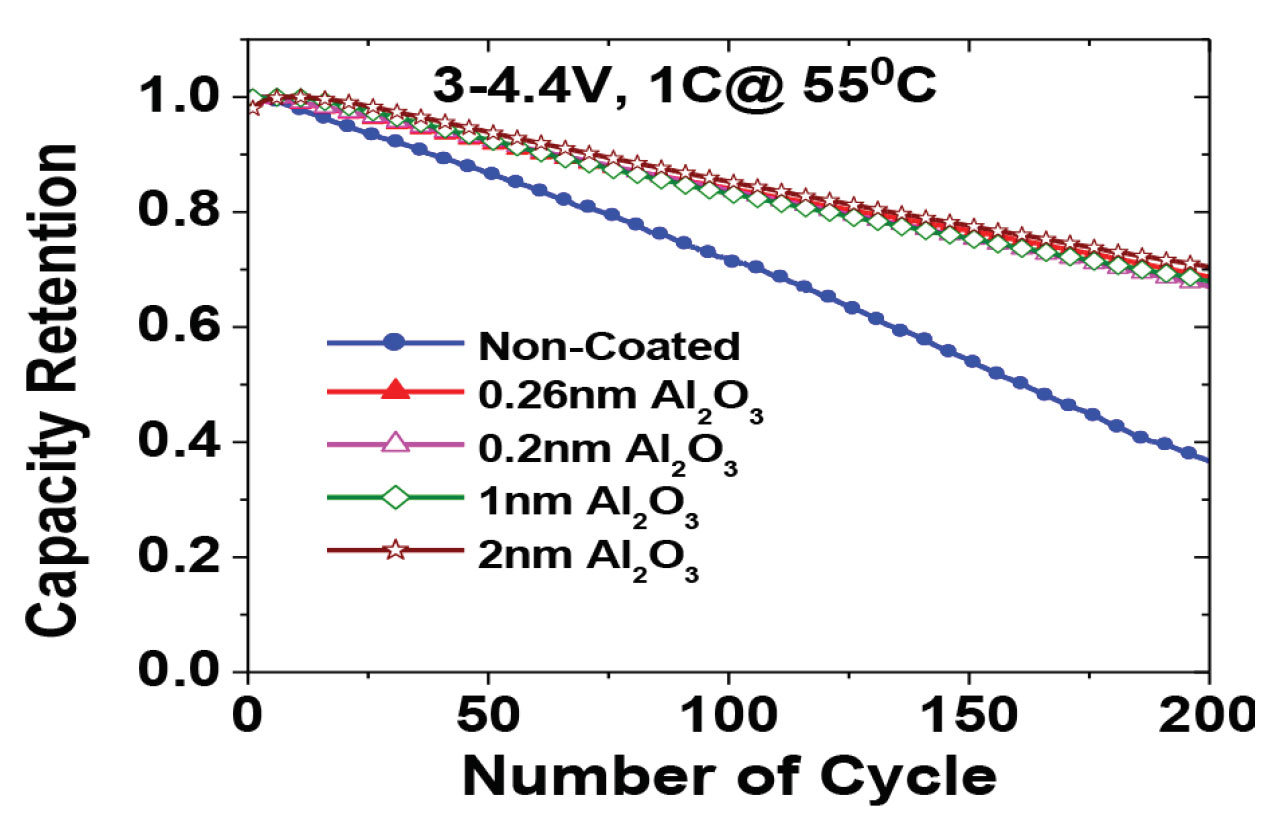
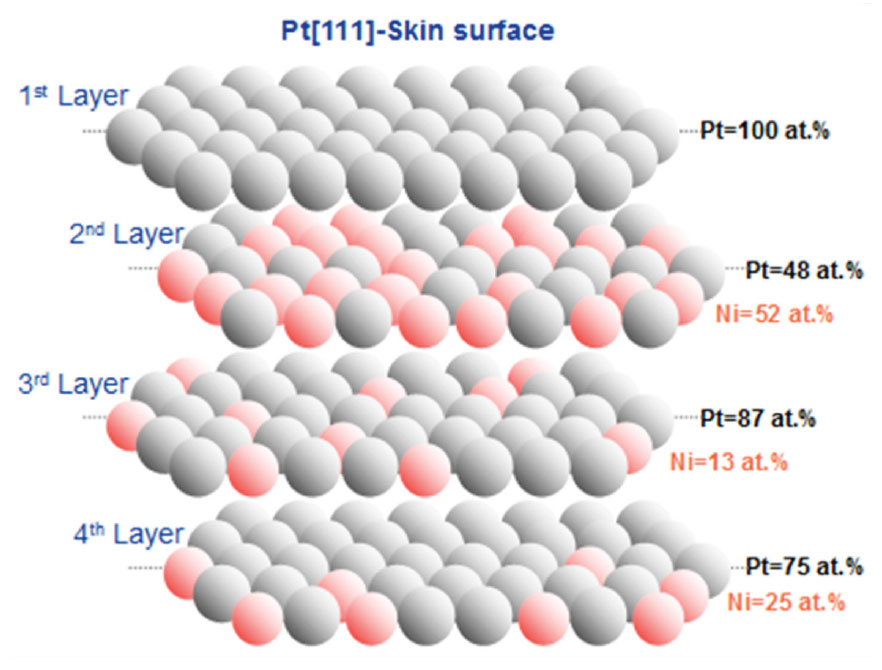
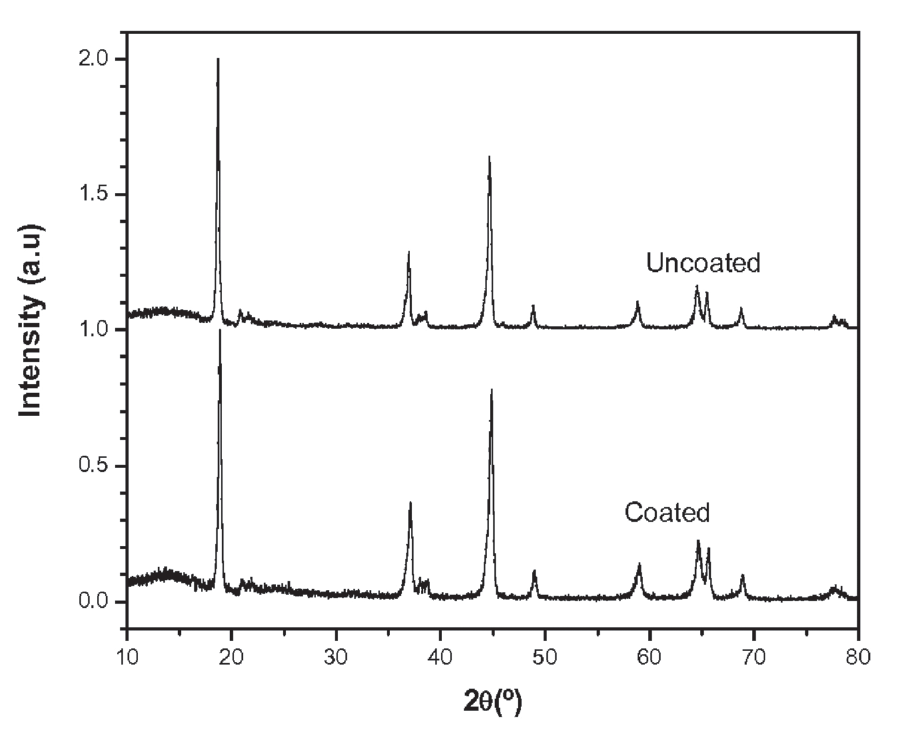
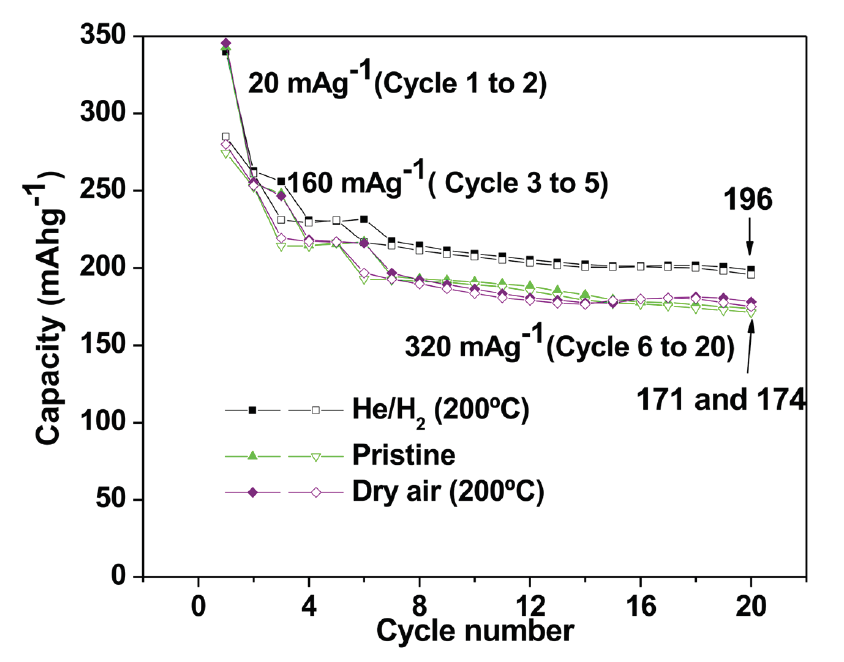
- Preparation of Carbon-Based Electrodes with High Thermal Conductivity for Battery Applications (ANL-IN-13-078)
The cathode and anode are in the form of electroactive sheets separated from each other by a membrane that is permeable to the electrolyte. One or more of the cathode and anode comprises two or more layers of carbon nanotubes, one of which layers includes electrochemically active nanoparticles and/or microparticles disposed therein or deposited on the nanotubes thereof. The majority of the carbon nanotubes in each of the layers are oriented generally parallel to the layers. Optionally, one or more of the layers includes an additional carbon material such as graphene, nanoparticulate diamond, microparticulate diamond, and a combination thereof.
Benefits
- Unique combination of diamond nanoparticles and other carbon materials
- Improves the ability to remove heat efficiently from the battery system