- Device and Method for Fluidizing and Coating of Ultrafine Particles (ANL-IN-11-048)
The Invention
An ultra thin surface coating composed of metal oxides that, when applied to granular electrode materials on a large scale, promises to solve the structural instability of electrode materials and the resulting rapid fade of cell capacity at high voltages and high temperatures in lithium-ion batteries.
Argonne’s innovation, a powder nanocoating technology using metal oxides, has the following features:
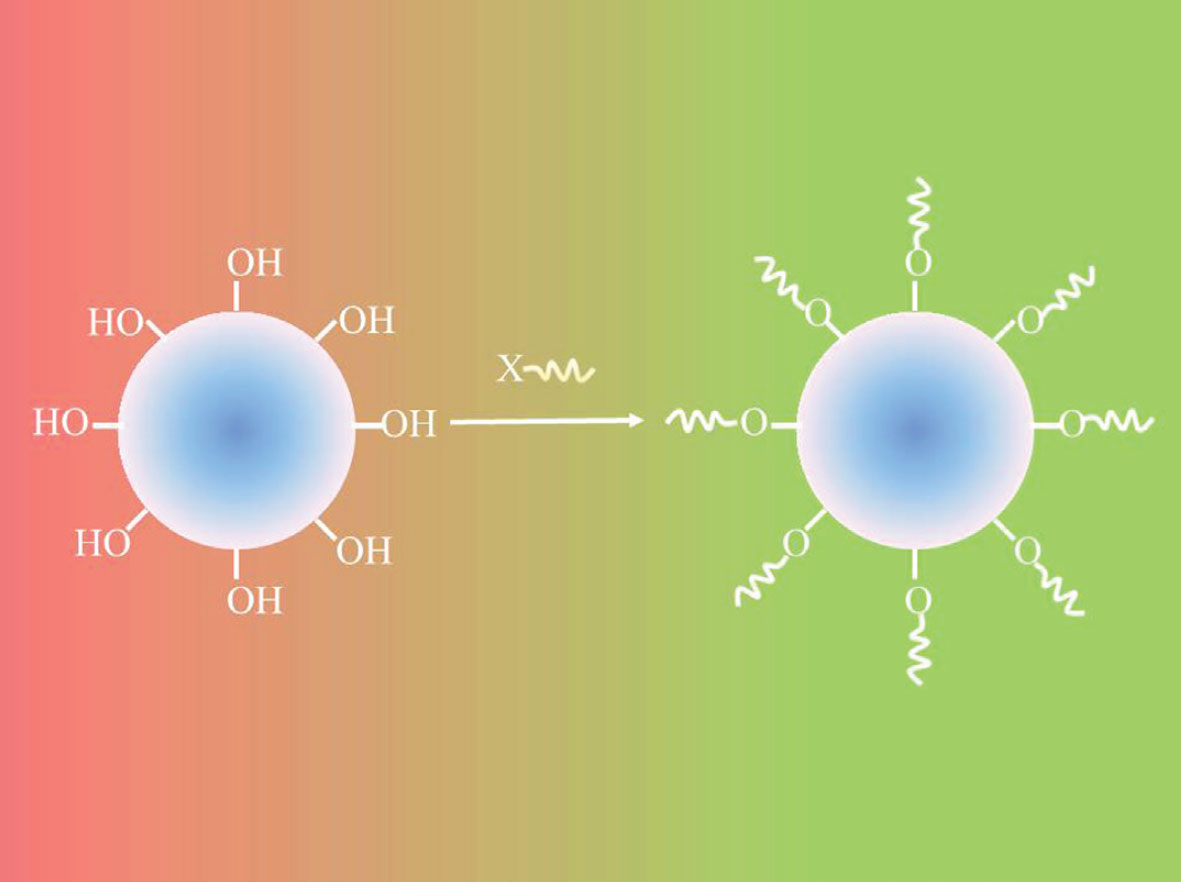
- Gas-phase surface chemical reactions;
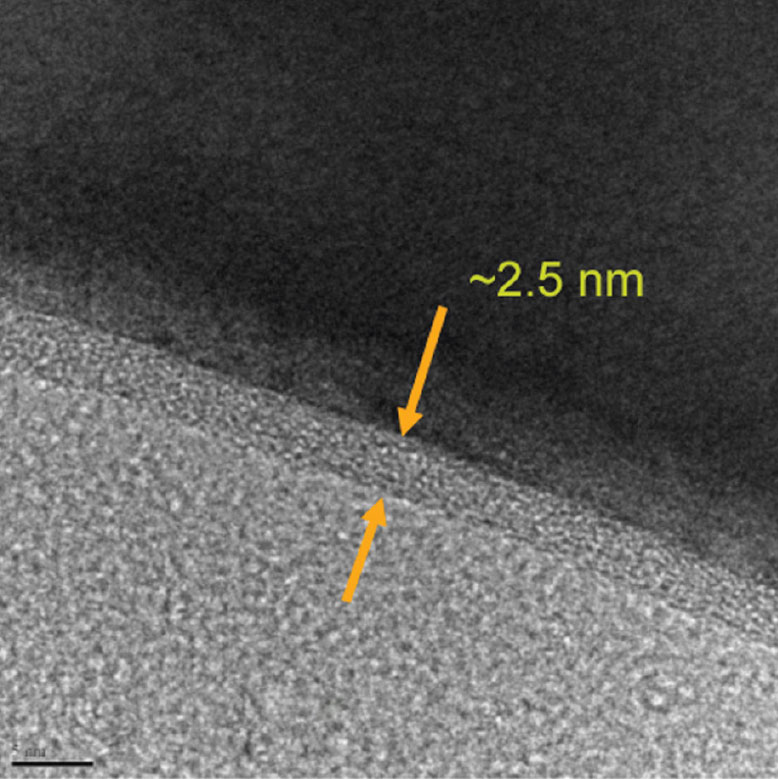
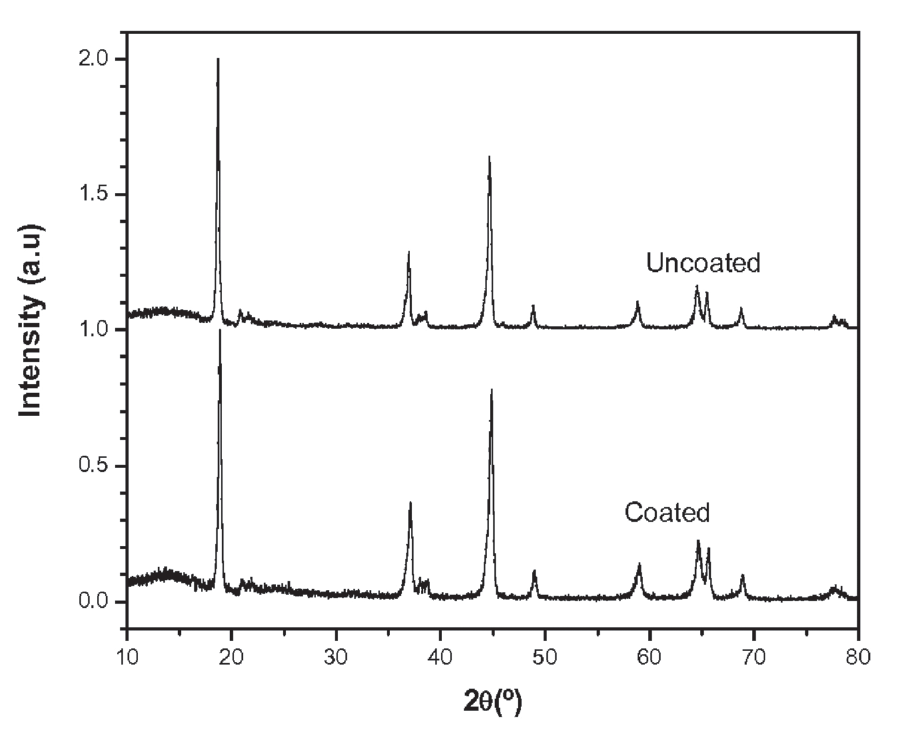
- A layer of extremely uniform metal oxide ultrathin film on granular cathode materials with precisely controlled surface morphology: smooth, conformal, and pin-hole free so that the electrode degradation reactions in the battery can be suppressed; and
- Film so ultra thin and precisely controlled in its thickness that the transfer of the charge across the electrode/electrolyte interface takes place with a very limited, or even a reduced, interface resistance.
In developing a surface coating for the electrodes of lithium-ion batteries, Argonne scientists sought to satisfy two requirements simultaneously:
- Create a uniform coating that will fully isolate electrodes from the electrolyte, and
- Create an ultra thin film that will allow the lithium ion and electron to easily tunnel without a large increase in impedance.
Conventional technologies have been unable to fulfill those requirements and have proved incapable of precisely controlling the coating film properties of film thickness and morphology. As a result, battery performance can be unstable.
Benefits
The new powder coating technology provides:
- Smooth fluidization of ultrafine powders via non-linear processing control;
- Online, real-time monitoring of powder fluidization status and surface chemical reaction;
- Well-controlled properties of the nanocoated film (conformity, thickness, and composition); and
- A novel process that is scalable, less energy-intensive, and at a lower cost.
Lithium-ion batteries made of these novel coated materials offer:
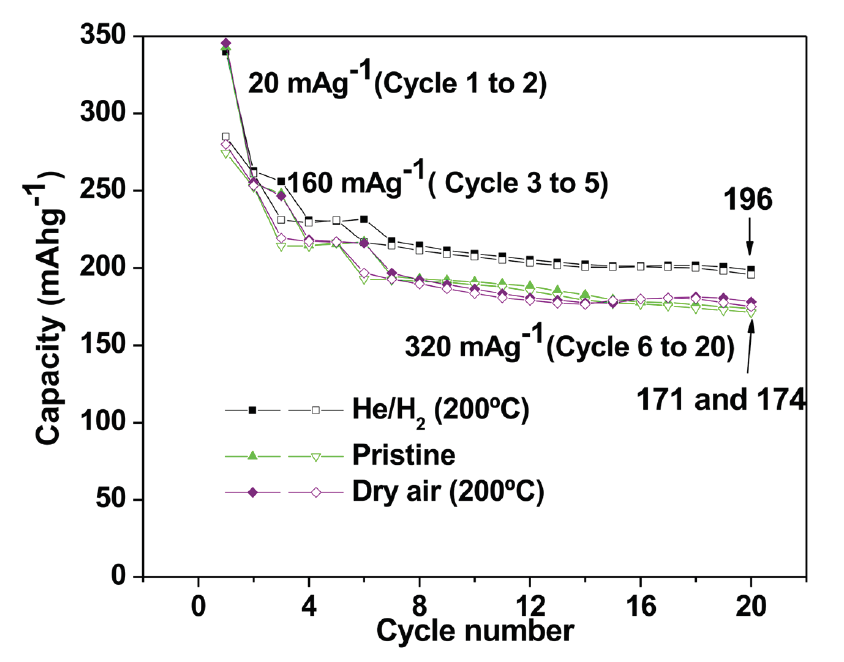
- Isolation of electrode from electrolyte, creating greater structural stability and effectively enhancing capacity retention;
- Greater stability;
- Longer lifespan;
- Higher energy/power densities;
- Greater safety; and
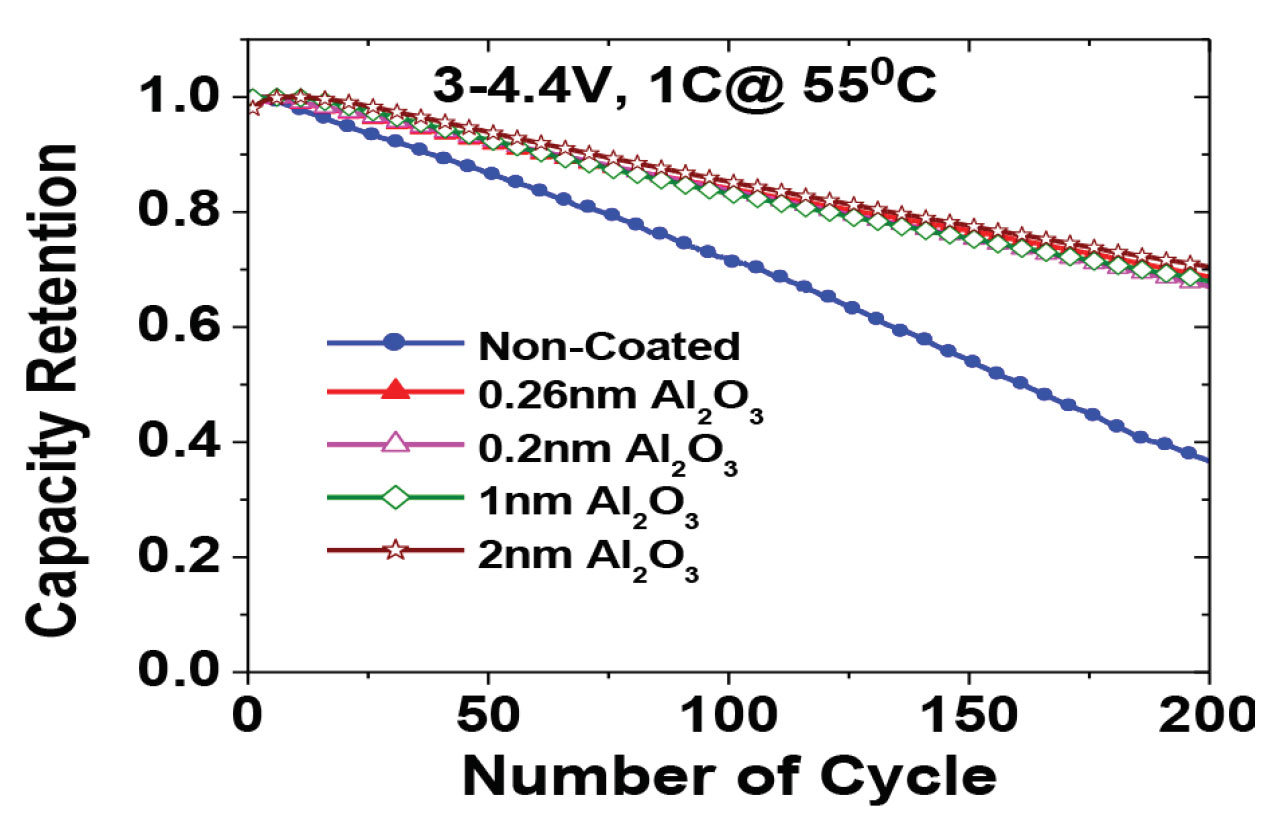
- Reduced cost and increased performance (figure 2) reliability.
Applications and Industries
- Hybrid electric vehicles
- Solar cells
- Ultracapacitors
- Cosmetics
Developmental Stage
Proof of concept. Lab scale has been demonstrated; small pilot scale up is on schedule.