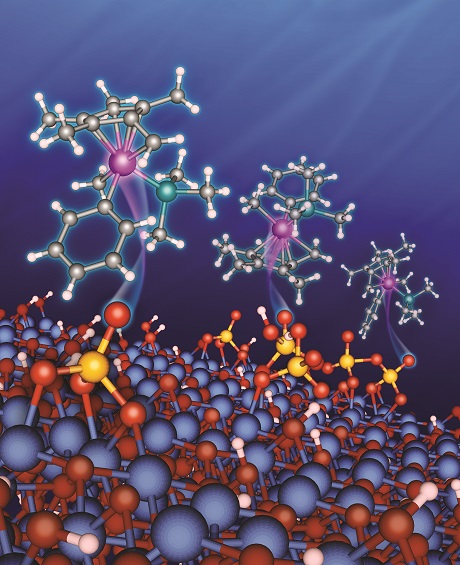
A key goal of the Catalysis Science Program at Argonne is to control the activity and selectivity of C–C bond formation and C–H bond activation in alkenes and light alkanes, via understanding of electronic cooperativity in supported “atomically-precise” catalysts. Specifically, we are developing catalyst systems where reactivity is dependent on the electronic communication between the active site and the support or promoter metal. To achieve this objective, we are studying two classes of catalytic reactions as a probe for these effects: (1) catalytic olefins upgrading (dimerization and metathesis) and (2) catalytic non-oxidative dehydrogenation of light alkanes. In addition to their relevance to the efficient use of the nation’s abundant shale gas reserves (C2-C4), these reactions also represent reaction networks for which an opportunity exists to selectively navigate the complex energy landscape through careful control of electronic structure and interactions. Importantly, the knowledge gained from these case studies will be broadly applicable to other reaction manifolds. In addition, multimetallic sub-nanometer nanoparticles have attractive properties in the context of thermal dehydrogenation; however, the mechanistic underpinnings for their increased activity and selectivity, as well as predictive principles for their precision synthesis, remain under-explored and are a primary focus in our program.
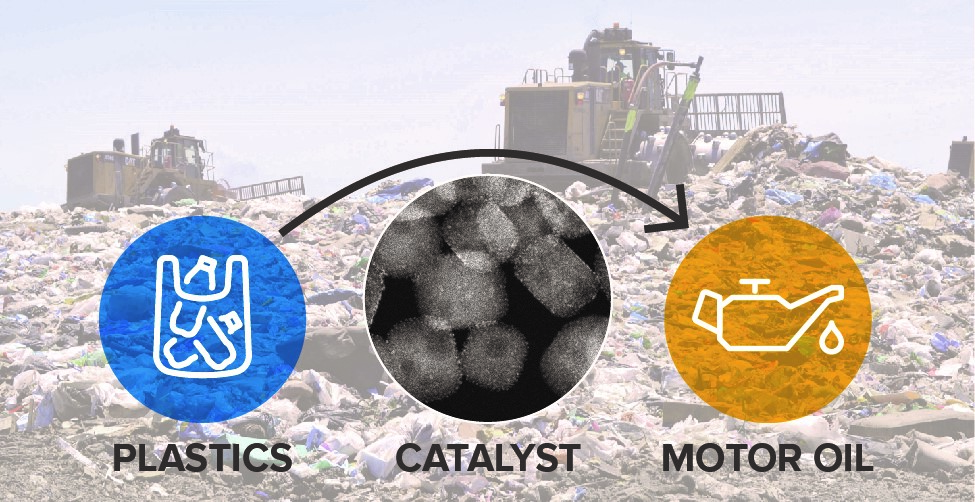
Another research goal is the upcycling of single-use polyethylene into high-quality liquid products. To that end, energy-rich polyethylene macromolecules are catalytically transformed into value-added products by hydrogenolysis using atomic layer deposition of platinum nanoparticles supported on SrTiO3 perovskite nanocuboids. Our approach for the transformation of high molecular weight polyethylene requires the C−C bonds to undergo selective hydrogenolysis to produce a narrow molecular weight distribution of high-quality liquid hydrocarbons.
The Catalysis Science Program team is organized into three complementary and highly integrated scientific areas (Synthesis and Catalysis, Advanced X-ray Characterization, and Computational Design). Each area provides important insight into C–C bond formation and C–H bond activation, while addressing fundamental questions about how “functionally homogeneous supported catalysts” influence catalytic properties.
The Catalysis Science Program takes advantage of the unique synthesis and characterization techniques and instrumentation available at the Chemical Sciences and Engineering division and Argonne user facilities including the Advanced Photon Source and Center for Nanoscale Materials for elucidating the dynamic behavior of the catalysts under reaction conditions and exploiting the chemical properties that influence catalyst performance. Computational quantum chemistry calculations for the development of activity-descriptor relationships to guide experiments are performed using the high-performance computing capabilities available at the Argonne Leadership Computing Facility. All of the information gained through these strategies is critical for structure-function correlations and will provide direction for future iterations to the rational design of functional alkane upgrading catalysts.
Selected publications:
- Chapovetsky, A.; Langeslay, R. R.; Celik, G.; Perras, F. A.; Pruski, M.; Ferrandon, M. S.; Wegener, E. C.; Kim, H.; Dogan, F.; Wen, J.; Khetrapal, N.; Sharma, P.; White, J.; Kropf, A. J.; Sattelberger, A. P.; Kaphan, D. M.; Delferro, M., Activation of Low-Valent, Multiply M–M Bonded Group VI Dimers toward Catalytic Olefin Metathesis via Surface Organometallic Chemistry. Organometallics 2020, 39, 1035–1045.
- Kaphan, D. M.; Ferrandon, M. S.; Langeslay, R. R.; Celik, G.; Wegener, E. C.; Liu, C.; Niklas, J.; Poluektov, O. G.; Delferro, M., Mechanistic Aspects of a Surface Organovanadium(III) Catalyst for Hydrocarbon Hydrogenation and Dehydrogenation. ACS Catal. 2019, 9, 11055-11066.
- Syed, Z. H.; Kaphan, D. M.; Perras, F. A.; Pruski, M.; Ferrandon, M. S.; Wegener, E. C.; Celik, G.; Wen, J.; Liu, C.; Dogan, F.; Goldberg, K. I.; Delferro, M. Electrophilic Organoiridium(III) Pincer Complexes on Sulfated Zirconia for Hydrocarbon Activation and Functionalization. J. Am. Chem. Soc. 2019, 141, 6325-6337.
- Camacho-Bunquin, J.; Ferrandon, M. S.; Sohn, H.; Kropf, A. J.; Yang, C.; Wen, J.; Hackler, R. A.; Liu, C.; Celik, G.; Marshall, C. L.; Stair, P. C.; Delferro, M., Atomically Precise Strategy to a PtZn Alloy Nanocluster Catalyst for the Deep Dehydrogenation of n-Butane to 1,3-Butadiene. ACS Catal. 2018, 8, 10058-10063 (DOI: 10.1021/acscatal.8b02794).
- Langeslay, R. R.; Kaphan, D. M.; Marshall, C. L.; Sattelberger, A. P.; Stair, P. C.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem Rev. 2018 (DOI: 10.1021/acs.chemrev.8b00245).
- Langeslay, R. R.; Sohn, H.; Hu, B.; Mohar, J. S.; Ferrandon, M.; Liu, C.; Kim, H.; Niklas, J.; Poluektov, O. G.; Alp, E. E.; Sattelberger, A. P.; Hock, A. S.; Delferro, M. Nuclearity Effects in Supported, Single-Site Fe(II) Hydrogenation Catalysts. Dalton Trans. 2018, 47, 10842–10846 (DOI:10.1039/C8DT02720J).
- Klet, R. C.; Kaphan, D. M.; Liu, C.; Yang, C.; Kropf, A. J.; Perras, F. A.; Pruski, M.; Hock, A. S.; Delferro, M., Evidence for Redox Mechanisms in Organometallic Chemisorption and Reactivity on Sulfated Metal Oxides. J. Am. Chem. Soc. 2018, 140, 6308−6316 (JACS May 23, 2018 Cover and JACS Spotlights; DOI: 10.1021/jacs.7b11981).
- Kaphan, D. M.; Klet, R. C.; Perras, F. A.; Pruski, M.; Yang, C.; Kropf, A. J.; Delferro, M. Surface Organometallic Chemistry of Supported Iridium(III) as a Probe for Organotransition Metal–Support Interactions in C–H Activation. ACS Catal. 2018, 8, 5363-5373 (DOI: 10.1021/acscatal.8b00855).
- Camacho-Bunquin, J.; Ferrandon, M.; Sohn, H.; Yang, D.; Liu, C.; Ignacio-de Leon, P. A.;Perras, F. A.; Pruski, M.; Stair, P. C.; Delferro, M. Chemo-Selective Hydrogenation of Nitro Compounds with Supported Organoplatinum(IV) on Zn(II)/SiO2 Catalyst. J. Am. Chem. Soc. 2018, 140, 3940−3951 (DOI: 10.1021/jacs.7b11981).
- Liu, C.; Camacho-Bunquin, J.; Ferrandon, M.; Savara, A.; Sohn, H.; Yang, D.; Kaphan, D. M.; Langeslay, R. R.; Ignacio-de Leon, P. A.; Liu, S.; Das, U.; Yang, B.; Hock, A. S.; Stair, P. C.; Curtiss, L. A.; Delferro, M. Development of Activity-Descriptor Relationship in Supported Metal Ion Hydrogenation Catalysts on Silica. Polyhedron 2018, 152, 73–83 (DOI: 10.1016/j.poly.2018.06.006).
- Sohn, H.; Camacho-Bunquin, J.; Langeslay, R. R.; Ignacio-de Leon, P. A.; Niklas, J.; Poluektov, O. G.; Liu, C.; Connell, J. G.; Yang, D.; Kropf, J.; Kim, H.; Stair, P. C.; Ferrandon, M.; Delferro, M. Supported Single-Site Organo-Vanadium(III) Catalyst on Silica for Hydrogenation of Alkenes and Alkynes. Chem. Commun. 2017, 53, 7325-7328 (invited for special issue: ChemComm Emerging Investigators Issue 2017; DOI: 10.1039/C7CC01876B).